Search
- Page Path
- HOME > Search
Original Article
- Clinical Study
- A Phase II Multi-Center, Non-Randomized, Parallel Group, Non-Inferiority Study to Compare the Efficacy of No Radioactive Iodine Remnant Ablation to Remnant Ablation Treatment in Low- to Intermediate-Risk of Papillary Thyroid Cancer: The MOREthyroid Trial Protocol
- Eun Kyung Lee, You Jin Lee, Young Joo Park, Jae Hoon Moon, Ka Hee Yi, Koon Soon Kim, Joo Hee Lee, Sun Wook Cho, Jungnam Joo, Yul Hwangbo, Sujeong Go, Do Joon Park
- Endocrinol Metab. 2020;35(3):571-577. Published online September 22, 2020
- DOI: https://doi.org/10.3803/EnM.2020.681
- 4,640 View
- 119 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
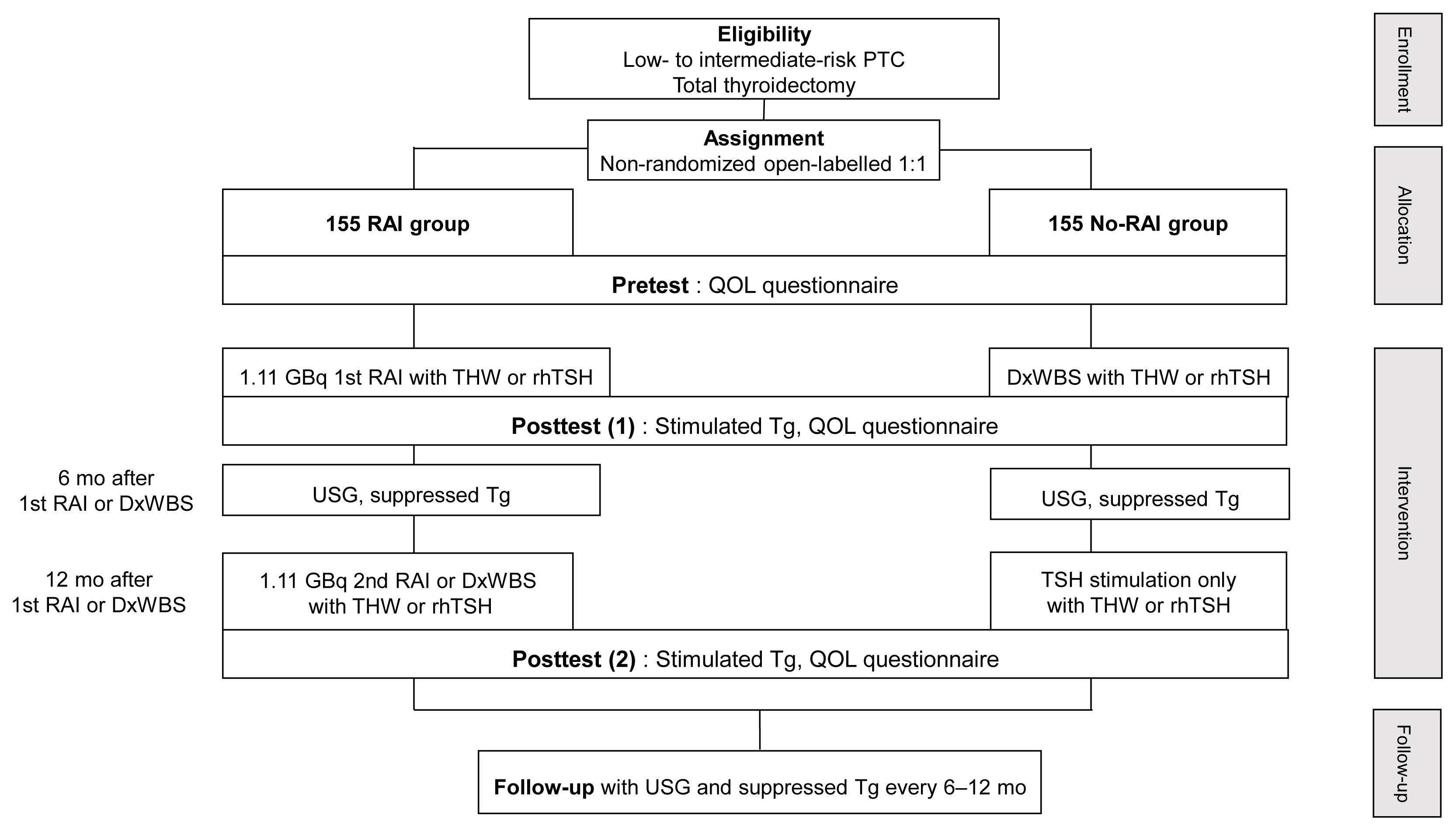
Radioactive iodine (RAI) remnant ablation is recommended in patients with papillary thyroid cancer (PTC) and extrathyroidal extension or central lymph node metastasis. However, there exists little evidence about the necessity of remnant ablation in PTC patients with low- to intermediate-risk, those have been increasing in recent decades.
Methods
This multicenter, prospective, non-randomized, parallel group clinical trial will enroll 310 eligible patients with low- to intermediate-risk of thyroid cancer. Inclusion criteria are patients who recently underwent total thyroidectomy for PTC with 3 or less tumors of size 1≤ to ≤2 cm with no microscopic extension and N0/x, or size ≤2 cm with microscopic extension and/or N1a (number of lymph node ≤3, size of tumor foci ≤0.2 cm, and lymph node ratio <0.4). Patients choose to undergo RAI ablation (131I, dose 1.1 GBq) or diagnostic whole-body scan (DxWBS) (131I or 123I, dose 0.074 to 0.222 GBq), followed by subsequent measurement of stimulated thyroglobulin (sTg) within 1 year. Survey for quality of life (QOL) will be performed at baseline and at 1 year after follow-up. The total enrollment period is 5 years, and patients will be followed up for 1 year. The primary endpoint is the non-inferiority of surgery alone to surgery with ablation in terms of biochemical remission (BCR) rate (sTg ≤2 ng/mL) without evidence of structural recurrence. The secondary endpoint was the difference of QOL.
Conclusion
This study will evaluate whether surgery alone achieves similar BCR and improved QOL compared to RAI ablation in patients with low- to intermediate-risk PTC within 1 year.


 KES
KES
 First
First Prev
Prev



